
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >High-Tech NEW Material >554-13-2

Product Details
|
General Description |
Lithium carbonate is a medication used to treat and prevent episodes of mania (abnormally elevated mood) in people with bipolar disorder. It works by reducing the activity of certain chemicals in the brain, including serotonin and dopamine. It is also used to reduce the frequency and severity of manic episodes in patients with bipolar disorder. Additionally, lithium carbonate is known to help reduce the risk of suicide in people with mood disorders. This medication is typically taken in capsule or tablet form and is usually prescribed alongside other treatments, such as therapy and other medications. It is important to use lithium carbonate as directed by a healthcare professional, as improper use can lead to serious side effects and complications. |
InChI:InChI=1/CH2O3.2Li/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
For the Li-N system samples were obtaine...
When solubilized in a hexacarboxamide cr...
Solid state and solution phase decomposi...
We report for the first time the synthes...
Li1-xNi1+xO2 was prepared by a polymeriz...
An excessive amount of CO2is the leading...
Pure monoclinic phase Li6Zr2O7 and rhomb...
Cells of the type Au/Li4SiO4/Au and Au/L...
Li5GaO4 was tested as a possibleCO2 capt...
Solid-state batteries offer higher energ...
A novel spectroscopic in situ light scat...
Lithium oxosilicate was synthesized via ...
Li8ZrO6 contains a high lithium content ...
Herein, the dependence of spinel LiMn2O4...
In the Li//2O-Ta//2O//5 system there are...
The controlled potential electrolysis of...
The evolution of Cu-18at%Li crystals, Cu...
The proton/lithium exchange property of ...
We demonstrate for the first time, a new...
This paper presents a general method of ...
Reduced CO2 species are key intermediate...
Alkali metal salts M2[1] (M=Li, Na) of d...
Olivine structure LiFePO4has attracted m...
The thermal decomposition of alkali (Li,...
Owing to the merits of high capacity and...
Hierarchical porous carbon (HPC) has att...
Abstract: Processes of the multi-stage d...
Methyl xanthates of the type M(SSC-OMe) ...

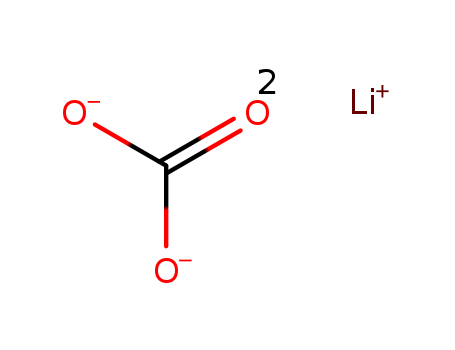
lithium carbonyl

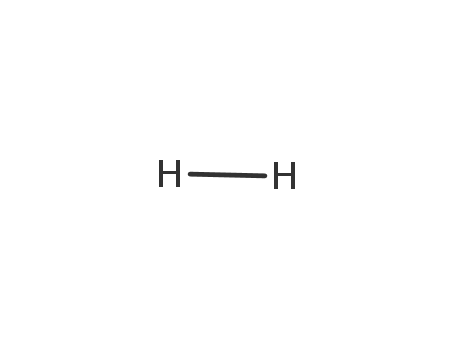
hydrogen

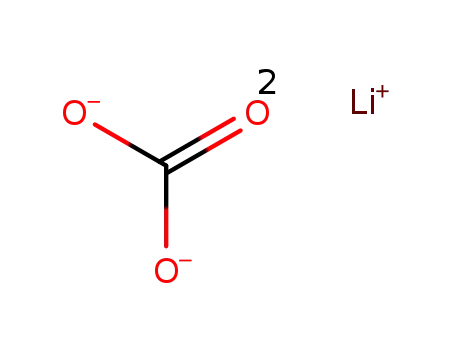
lithium carbonate

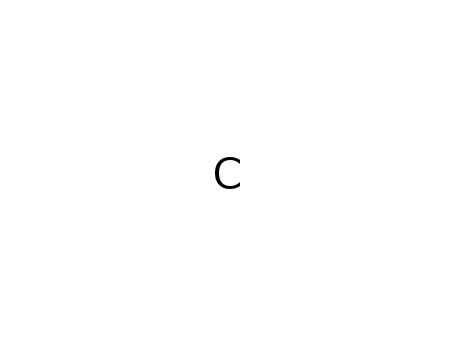
pyrographite
| Conditions | Yield |
|---|---|
|
With
H2O;
In
water;
on treating with water vigorous explosion with ignition of the gaseous products;;
|
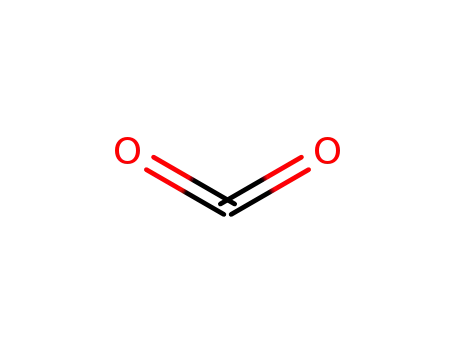
carbon dioxide

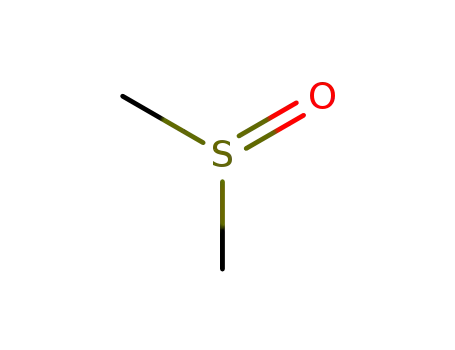
dimethyl sulfoxide

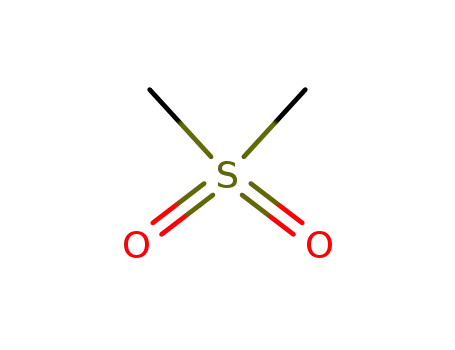
dimethylsulfone


lithium carbonate
| Conditions | Yield |
|---|---|
|
With
lithium peroxide;
at 25 ℃;
for 48h;
under 760.051 Torr;
|
90 %Spectr. |
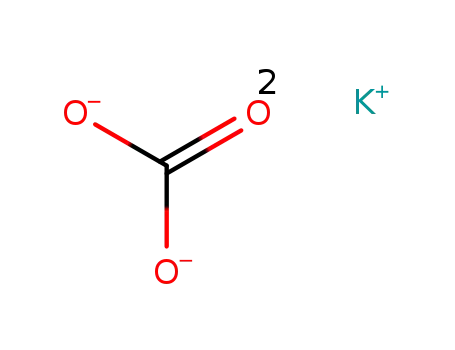
potassium carbonate
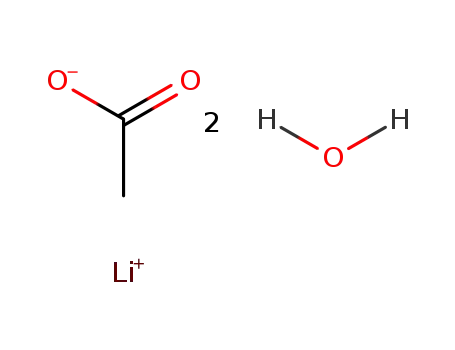
lithium acetate dihydrate
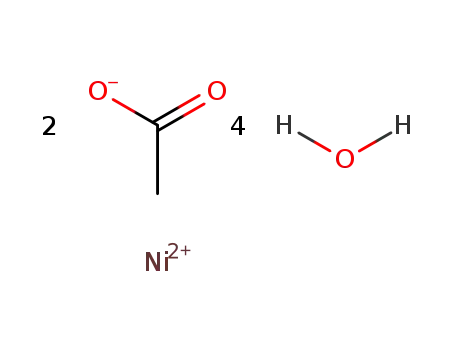
nickel(II) acetate tetrahydrate
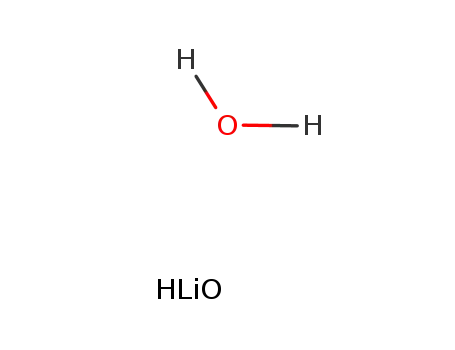
lithium hydroxide monohydrate
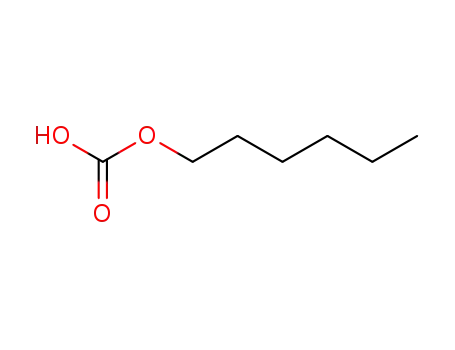
hexylcarbonate
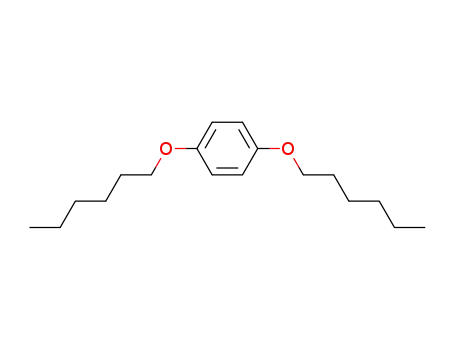
1,4-dihexyloxybenzene
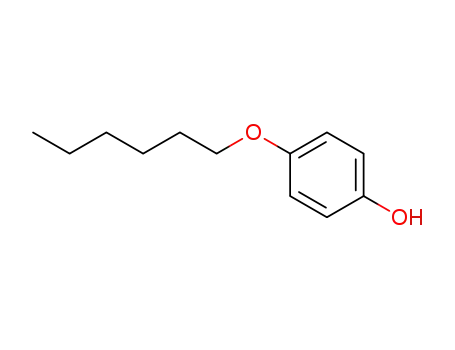
4-(hexyloxy)phenol
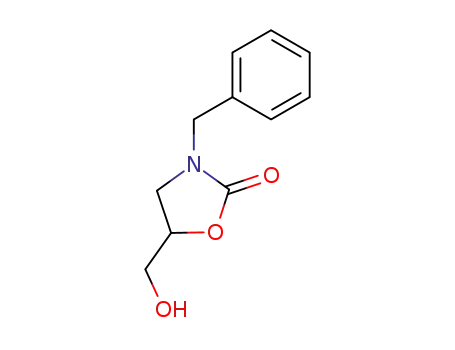
(RS)-3-benzyl-5-(hydroxymethyl)-1,3-oxazolidin-2-one