
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >High-Tech NEW Material >13770-96-2
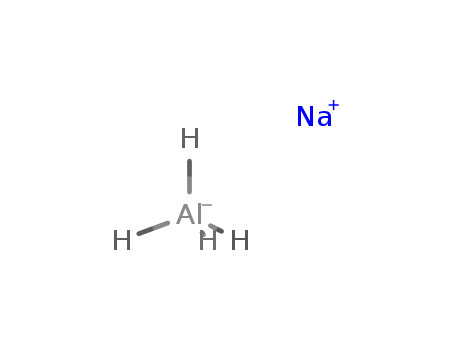
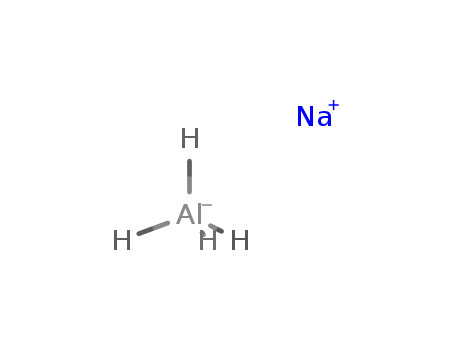
Product Details
|
Preparation |
Sodium aluminium hydride can be synthesized by reacting powdered sodium hydride and aluminium with hydrogen at 200 °C under high pressure (<100 atm). A catalyst, like triethylaluminium is used. |
|
Air & Water Reactions |
Highly flammable. Does not react in dry air at room temperature. Very sensitive to moisture. Ignites or explodes on contact with water or moist air [NSC Nat. Safety News 77(2):37-40 1958]. Reacts with water to form sodium hydroxide, a corrosive material and hydrogen, a flammable gas. The heat of reaction may be sufficient to ignite the hydrogen. |
|
Reactivity Profile |
Sodium aluminium hydride is a strong reducing agent. Similar in reactivity to lithium aluminum hydride. May react violently with oxidizing agents. An explosion occurred during its preparation from sodium and aluminum in a medium of tetrahydrofuran [Chem. Eng. News 39(40):57 1961]. At elevated temperatures, the hydride reduces carbon dioxide or sodium hydrogen carbonate to methane and ethane. These gases are the explosive products formed when CO2 extinguishers have been used during hydride fires. 1:1 complexes with ether or dimethylamine and various tetrazoles are explosive. Tetrazoles include, 2-methyl, 2-ethyl, 5-ethyl, 2-methyl-5-vinyl, 5-amino-2-ethyl, etc. [US Pat. 3 396 170, 1968]. |
|
Hazard |
Severe fire and explosion risk in contact with oxidizing agents and water, forms caustic and irritating compounds. |
|
Health Hazard |
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. |
|
Fire Hazard |
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard. |
|
Safety Profile |
Flammable when exposed to heat or flame. May ignite and explode on contact with water. Reacts violently with tetrahydrofuran when heated. When heated to decomposition it emits toxic fumes of NanO. See also ALUMINUM COMPOUNDS and HYDRIDES. |
|
Potential Exposure |
Used in chemical synthesis |
|
Shipping |
UN2835 Sodium aluminum hydride, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. |
|
Incompatibilities |
A strong reducing agent. Very sensitive to moisture; reaction with water may cause fire or explosion. Also incompatible with iron, aluminum, and zinc in the presence of water. Hydrides are incompatible with acids, alcohols, amines, and aldehydes. Violent reaction, possibly explosive, on contact with water, air, oxidizers, acids, alcohols, and ethers. May ignite spontaneously in moist air. Does not react in dry air at room temperature. Reacts with water to form corrosive NaOH and flammable hydrogen gas. The heat of reaction may be sufficient to ignite. |
|
General Description |
A white crystalline solid. Density 1.24 g / cm3. If spread over a large flat combustible area, friction can ignite the material. Soluble in tetrahydrofuran. |
InChI:InChI=1/Al.Na.4H/q-1;+1;;;;/rAlH4.Na/h1H4;/q-1;+1
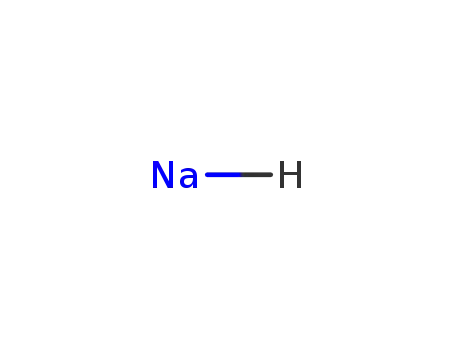
sodium hydride

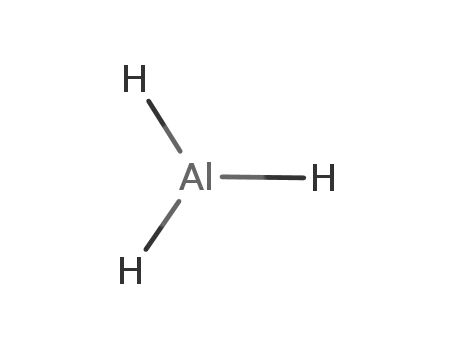
aluminium hydride

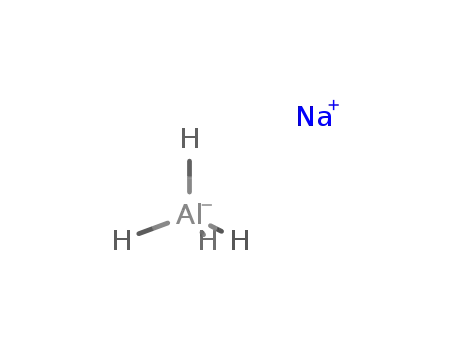
sodium tetrahydridoaluminate
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
components solns. mixing, crystn. on abs. ether diln.;
|
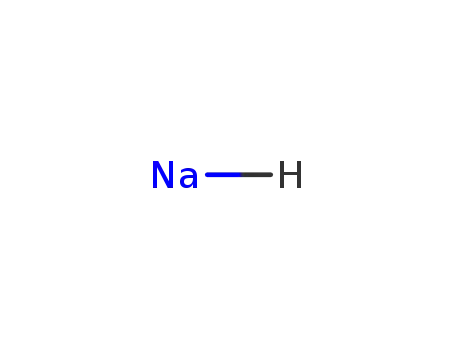
sodium hydride

aluminium hydride