
Antioxidant BHT 264
CAS:128-37-0
Purity:99%
Contact Now
We will contact you as soon as possible
Your Location:Home >Products >Pharmaceutical API >942-93-8

Product Details
|
General Description |
5-chloro-1-phenylpentan-1-one, also known as 5-Cl-PP, is an organic compound with the chemical formula C11H13ClO. It is a clear, colorless liquid with a slightly sweet odor that is used as a precursor in the synthesis of pharmaceuticals and other organic compounds. 5-Cl-PP is also known to have various industrial uses, such as in the production of fragrances and flavorings. Its chemical structure contains a chlorine atom and a phenyl group, making it a versatile building block for the creation of a wide range of chemical compounds. Due to its potential for use in the production of various products, 5-chloro-1-phenylpentan-1-one is an important compound in the field of organic chemistry and chemical synthesis. |
InChI:InChI=1/C11H13ClO/c12-9-5-4-8-11(13)10-6-2-1-3-7-10/h1-3,6-7H,4-5,8-9H2
Alkylsilyl peroxides were generated in s...
An efficient copper-catalyzed radical ri...
A Ni-catalyzed direct C-H alkylation ofN...
A Cu-catalyzed O-alkylation of phenol de...
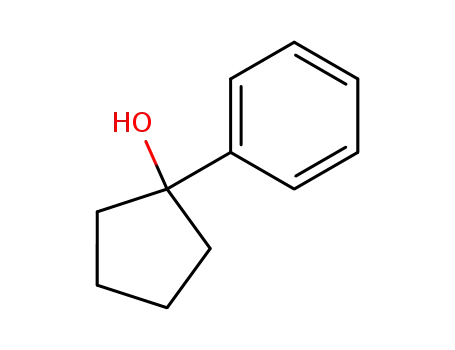
1-phenylcyclopentanol


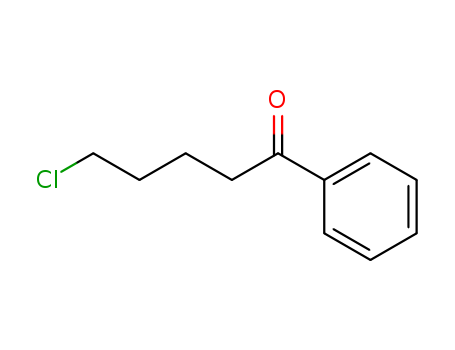
5-Chloro-1-phenyl-1-pentanone
| Conditions | Yield |
|---|---|
|
With
1,10-Phenanthroline; tert-butylhypochlorite; silver trifluoromethanesulfonate;
In
acetonitrile;
at 20 ℃;
for 48h;
regioselective reaction;
Inert atmosphere;
Schlenk technique;
|
80% |
|
Multistep reaction;
(i) aq. NaOCl, AcOH, (ii) (heating);
|
|
|
With
tetra(n-butyl)ammonium hydrogensulfate; hypochloric acid;
In
dichloromethane; chlorobenzene;
at 20 ℃;
for 0.5h;
pH=8.6 - 9.6;
|
|
|
Multi-step reaction with 2 steps
1: dihydrogen peroxide; sulfuric acid / water / 18 h / 0 - 20 °C / Inert atmosphere
2: copper(l) iodide; chloro-trimethyl-silane; triethylamine / N,N-dimethyl-formamide / 12 h / 28 °C / Inert atmosphere
With
copper(l) iodide; chloro-trimethyl-silane; sulfuric acid; dihydrogen peroxide; triethylamine;
In
water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: dihydrogen peroxide; sulfuric acid / water; dichloromethane / 12 h / 0 - 25 °C / Schlenk technique; Inert atmosphere
2: copper diacetate; hydrogenchloride / 1-methyl-pyrrolidin-2-one; water / 2 h / 25 °C / Schlenk technique; Inert atmosphere; Sealed tube
With
hydrogenchloride; sulfuric acid; dihydrogen peroxide; copper diacetate;
In
1-methyl-pyrrolidin-2-one; dichloromethane; water;
|
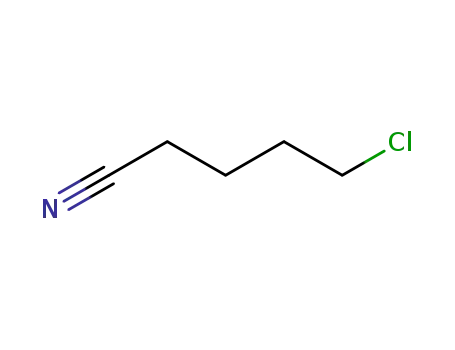
δ-chlorovaleronitrile

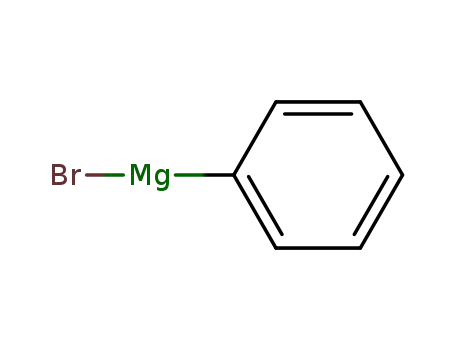
phenylmagnesium bromide


5-Chloro-1-phenyl-1-pentanone
| Conditions | Yield |
|---|---|
|
δ-chlorovaleronitrile; phenylmagnesium bromide;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 12h;
With
hydrogenchloride; water;
In
tetrahydrofuran;
for 3h;
Reflux;
|
81% |
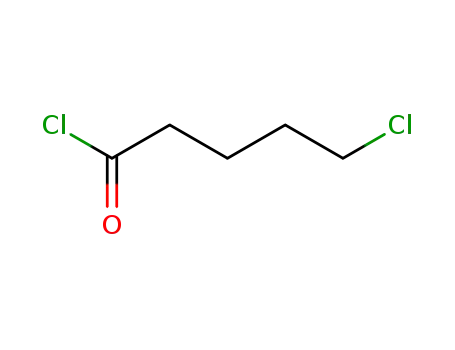
5-Chlorovaleroyl chloride

diphenylzinc
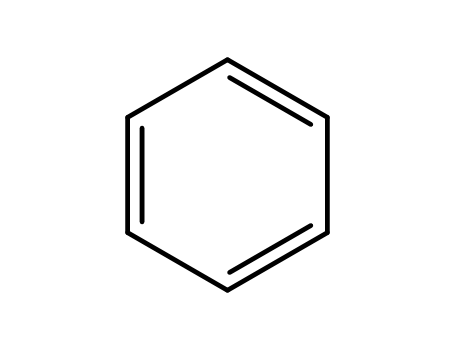
benzene

δ-chlorovaleronitrile
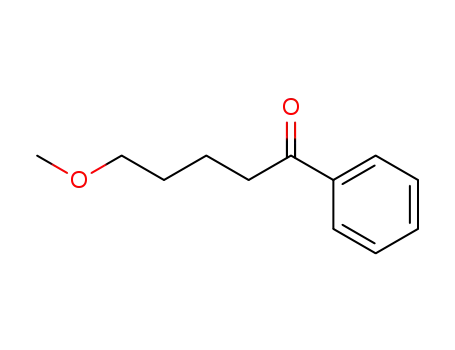
5-methoxy-1-phenyl-pentan-1-one
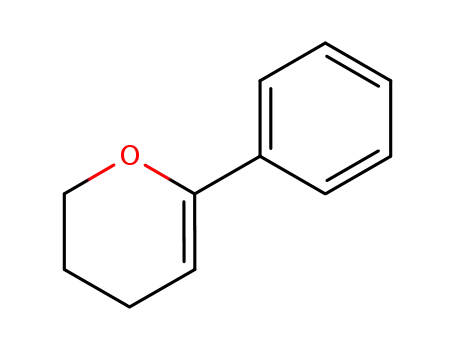
6-phenyl-3,4-dihydro-2H-pyran
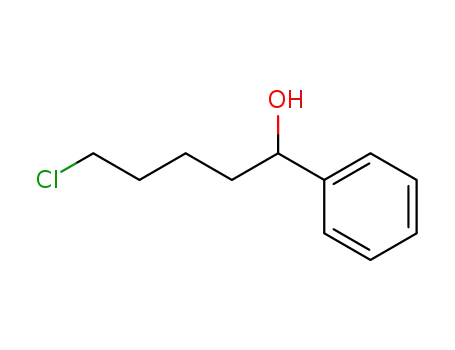
1-phenyl-5-chloropentan-1-ol
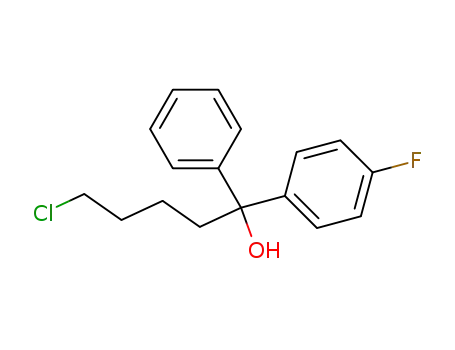
dl-5-Chlor-1-(4-fluorphenyl)-1-phenyl-pentanol